曾工致力于各类电子电器产品的国际国内认证、EMC整改,欢迎各位询价,提供专业服务,解决客户痛点!专治各种产品不合格!
电话:139 2899 3907 邮箱:info@emc.wiki
Covid 19 Suspicious Sertificates For PPE
欧洲安全联盟发布PPE的可疑证书
每个人都在非常努力地工作,并且有最好的意愿,为卫生保健工作者和参与COVID-19危机的其他人提供必要的PPE。
遗憾的是,我们从不同来源获悉了用作PPE CE标记(包括 FFP2/FFP3口罩和眼睛保护罩)的"证书",而这些"证书"没有法律价值,不能用作符合性评估的结论。
鉴于当前的卫生危机,欧盟委员会发布了关于合格评定和市场监督的 (EU) 2020/403建议。这允许成员国允许将个人防护装备投放到合格评定程序尚未完全完成的市场。这仅适用于与危机相关的产品,这些产品是在危机期间由当局为保健部门购买的。但这并不意味着产品不必符合 PPE 法规中定义的适用的基本健康和安全要求。
特别点名了,CELAB, ICR Polska (see update 31/03/2020 below), ISET, ECM, NPS, CIC, Amtre Veritas and GTS.
可疑证书清单
http://www.iset-italia.eu/index/service/fal.html
Suspicious certificates for PPE
Everybody is working very hard and with the best intentions to make the necessary PPE available to the healthcare workers and other people involved in the fight against the COVID-19 crisis.
Unfortunately, we are informed from different sources about ‘certificates’ used as basis for CE marking of PPE (including FFP2/FFP3 masks and eye protection), while these ‘certificates’ have no legal value and can not be used as conclusion of conformity assessment.
So far we have seen ‘certificates’ on letterhead of CELAB, ICR Polska (see update 31/03/2020 below), ISET, ECM, NPS, CIC, Amtre Veritas and GTS. It is not clear if these documents have actually been issued by the organisations mentioned and ESF is not accusing them of doing so. Indeed ISET has on their website a list of fake certificates, where one of the examples received is mentioned as false (see http://www.iset-italia.eu/index/service/fal.html, file named “Falsa attestazione-certificato kn95.pdf).
We have the impression that manufacturers outside the EU (and probably even ‘newcomers’ and importers in the EU) are not entirely familiar with the EU Legislation on PPE and thus believe that by paying the ‘certificate’ from such an organisation, they are fully in compliance with the EU legislation. And most likely, also on the side of the customers (including health authorities), the knowledge about the exact requirements of the EU legislation is lacking and thus they judge those documents as accurate. Hopefully the products offer at least the protection that is claimed in the documents, even if there is no solid proof of that.
Attached some examples from different institutes
- 2 examples of ICR Polska - see update 31/03/2020 below
- 2 examples of CELAB
- 1 example of ISET (mentioned on the website of ISET as false)
- 1 example of ECM (also picture of mask with identification number of the notified body ECM next to CE - ECM is not a notified body for PPE and thus this marking is not valid)
- 1 example of NPS
- 1 example of CIC
- 1 example of Amtre Veritas
- 1 example of GTS (document title is 'declaration of conformity')
At this moment, it is clear that the priority is to get as much as possible masks (and other relevant PPE) into the EU so that the healthcare workers can be protected. On the other hand, it cannot be accepted that sub-standard masks which offer not the claimed protection are made available to healthcare workers that are now at high risk and thus deserve correct protection.
Protective masks (like FFP2/FFP3) are PPE of category III according to the Regulation (EU)2016/425. This means that the conformity assessment included a type examination by a notified body, resulting in a ‘EU type examination certificate’ as well as production follow-up by a notified body (random checks or system audit). This results in CE marking with the number of the notified body responsible for the production follow-up next to the CE marking. The manufacturer is obliged to issue the EU Declaration of Conformity, which must accompany (at list via a weblink) the PPE, together with the instructions for use.
Given the current health crisis, the EU Commission published Recommendation (EU) 2020/403 on conformity assessment and market surveillance. This allows for member states to allow PPE being placed on the market for which the conformity assessment procedure has not fully been finished. This is only for products relevant to the crisis, bought for the duration of the crisis by authorities for the health care sector. But this does not mean that the products do not have to be in compliance with the applicable essential health and safety requirements defined in the PPE Regulation.
PPE entering the normal distribution chain however, still have to be fully compliant with the Regulation.
For everyone involved : check that ‘certificates’ you receive for the PPE are correctly titled ‘EU type examination certificate’ and that they are issued by a competent Notified Body (meaning certainly based in the EU, including EFTA and Turkey). The identification number of the Notified Body has to be included in the EU type examination certificate.
If you have doubts about the Notified Body, you can check the NANDO datebase (https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=155501) where you will find also the competences of the notified bodies (not all PPE notified bodies are competent for all types of PPE).
If you have doubts about the EU type examination certificate, do not hesitate to contact the notified body concerned with the question if the certificate is genuine (some of the notified bodies have a tool on their website to check certificates).
You can also contact the national organisation of PPE suppliers in your country (see the effective members of ESF for contact information).
For some more information on how to recognise ‘misleading’ or ‘fake’ certificates, see also the ESF Q&A 87 and 88
The China National Accreditation Service (CNAS for Conformity Assessment) has published a list of laboratories accredited for testing of masks (protective and medical) on their website. Remark : Chinese accredited laboratories are certainly not Notified Body for respiratory protection and thus cannot issue EU Type Examination Certificates, they can perform the testing according to the tests for which they are accredited (in the vast majority of cases this is only to the Chinese standards, not to the EN standards). click here for the list of CNAS
update 31/03/2020 : ICR Polska placed a message on their homepage concerning their voluntary certificates (see https://icrpolska.com/) :
Due to the increased interest in obtaining a certificate of compliance with the standards harmonized with the European Commission Regulation on personal protective equipment No. 2016/425, we would like to inform that ICR Polska Co, Ltd. no longer accepts applications for certification on this scope.
At the same time, we explain that, in accordance with the content of issued certificates, the assessments carried out so far are of a voluntary nature and are not equivalent to the mandatory conformity assessment procedures carried out by Notified Bodies authorized to the aforementioned Regulation. These certificates do not confirm conformity with all essential requirements of Regulation No. 2016/425 relating to the product.
annexes to the article
- text of the article in pdf format (the list of examples is updated with additional received examples on the webpage, but not yet in the pdf version of the text)
- example Celab 1
- example Celab 2
- example ECM
- picture mask with number of notified body ECM while ECM is not notified for PPE, so invalid marking
- example ICR Polska 1
- example ICR Polska 2
- example ISET
- example NPS
- example CIC
- example Amtre Veritas (certificate of compliance for safety goggles)
- example GTS
- link Q&A 87
- link Q&A 88
中国CNAS认可口罩(保护和医疗)检测的实验室名单
中国国家认可局(CNAS 合格评定)在其网站上公布了认可口罩(保护和医疗)检测的实验室名单
https://www.cnas.org.cn/english/photonews/03/902316.shtml
Scope of accreditation for some laboratories may be limited based on relevant standards, further information is available at https://las.cnas.org.cn/LAS_FQ/publish/externalQueryL1En.jsp
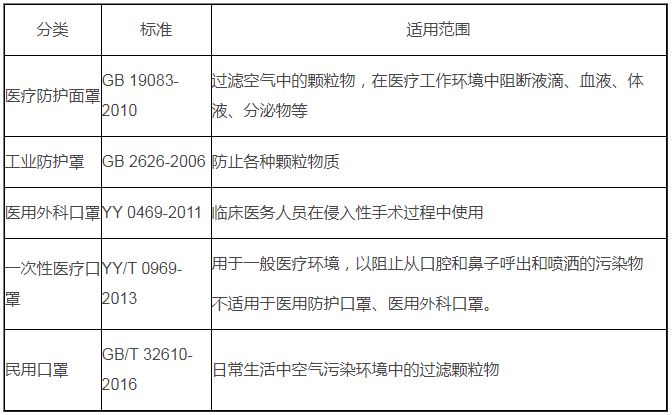
==中国口罩的分类和适用标准==测试标准及其适用的口罩范围如下:
a) GB 19083-2010 适用于医疗防护罩(GB 指强制性国家标准);
b) GB 2626-2006 用于工业防护罩;
c) YY 0469-2011 适用于医用外科口罩(YY 指制药行业的强制性标准);
d) YY/T 0969-2013 适用于一次性医疗口罩(YY/T 指制药行业推荐标准);
e) GB/T 32610-2016 适用于民用口罩(GB/T 指建议的国家标准)。